著名医師による解説が無料で読めます
すると翻訳の精度が向上します
背景:ブラックボックスの警告は、食品医薬品局が販売された処方薬の発行ができるという最も強力な安全性の警告を表しています。いくつかのブラックボックス警告は、深刻な、おそらく生命を脅かす効果のリスクが増加しているため、特定の薬物の採用に対して推奨されます。 目的:ブラックボックス警告で言及されている禁忌コメディケーションに関する3つの主要な薬物相互作用スクリーニングプログラムの存在下、臨床重症度スコアレベル、およびアラートコンテンツの存在下での合意のレベルを決定するため。 方法:現在販売されている処方薬の処方情報を、禁忌薬物の組み合わせに言及したブラックボックス警告を使用してレビューしました。We selected the drug interaction databases Facts & Comparisons 4.0, MICROMEDEX DRUG-REAX, and Lexi-Comp Lexi-Interact to evaluate the interactions.Discrepancies in the inclusion of interactions and level of agreement in clinical severity scores and level of documentation ratings for each interaction were assessed, using descriptive statistics, Spearman's correlation coefficient, Kendall-Stuart tau-c, and Cronbach's alpha. RESULTS: We identified 11 drugs with black box warnings that contained information on 59 unique contraindicated drug combinations, only 68% of which were covered by any source.Lexi-Compは、ほとんどの相互作用(n = 29)と薬物分解が最も少ない(n = 18)を検出しました。3つのデータベースすべてで、禁忌または潜在的に生命を脅かすものとして検出および評価された薬物の組み合わせのみが検出されました。The severity scores and level of documentation ratings varied widely. CONCLUSIONS: There are discrepancies among major drug interaction screening programs in the inclusion, severity, and level of documentation of contraindicated drug combinations mentioned in black box warnings. Further studies could explore the implications of these inconsistencies, particularly with regard to the integration of black box warning information in clinical practice. Clinicians should consult multiple drug resources to maximize the potential for detecting a potentially severe drug interaction.
背景:ブラックボックスの警告は、食品医薬品局が販売された処方薬の発行ができるという最も強力な安全性の警告を表しています。いくつかのブラックボックス警告は、深刻な、おそらく生命を脅かす効果のリスクが増加しているため、特定の薬物の採用に対して推奨されます。 目的:ブラックボックス警告で言及されている禁忌コメディケーションに関する3つの主要な薬物相互作用スクリーニングプログラムの存在下、臨床重症度スコアレベル、およびアラートコンテンツの存在下での合意のレベルを決定するため。 方法:現在販売されている処方薬の処方情報を、禁忌薬物の組み合わせに言及したブラックボックス警告を使用してレビューしました。We selected the drug interaction databases Facts & Comparisons 4.0, MICROMEDEX DRUG-REAX, and Lexi-Comp Lexi-Interact to evaluate the interactions.Discrepancies in the inclusion of interactions and level of agreement in clinical severity scores and level of documentation ratings for each interaction were assessed, using descriptive statistics, Spearman's correlation coefficient, Kendall-Stuart tau-c, and Cronbach's alpha. RESULTS: We identified 11 drugs with black box warnings that contained information on 59 unique contraindicated drug combinations, only 68% of which were covered by any source.Lexi-Compは、ほとんどの相互作用(n = 29)と薬物分解が最も少ない(n = 18)を検出しました。3つのデータベースすべてで、禁忌または潜在的に生命を脅かすものとして検出および評価された薬物の組み合わせのみが検出されました。The severity scores and level of documentation ratings varied widely. CONCLUSIONS: There are discrepancies among major drug interaction screening programs in the inclusion, severity, and level of documentation of contraindicated drug combinations mentioned in black box warnings. Further studies could explore the implications of these inconsistencies, particularly with regard to the integration of black box warning information in clinical practice. Clinicians should consult multiple drug resources to maximize the potential for detecting a potentially severe drug interaction.
BACKGROUND: Black box warnings represent the strongest safety warning that the Food and Drug Administration can issue for a marketed prescription drug. Some black box warnings recommend against coadministration of specific medications due to an increased risk for serious, perhaps life-threatening, effects. OBJECTIVE: To determine the level of agreement in presence, clinical severity scores level of documentation ratings, and alert content among 3 leading drug interaction screening programs with regard to contraindicated comedications that are mentioned in black box warnings. METHODS: We reviewed the prescribing information for currently marketed prescription drugs with a black box warning that mentioned a contraindicated drug combination. We selected the drug interaction databases Facts & Comparisons 4.0, MICROMEDEX DRUG-REAX, and Lexi-Comp Lexi-Interact to evaluate the interactions. Discrepancies in the inclusion of interactions and level of agreement in clinical severity scores and level of documentation ratings for each interaction were assessed, using descriptive statistics, Spearman's correlation coefficient, Kendall-Stuart tau-c, and Cronbach's alpha. RESULTS: We identified 11 drugs with black box warnings that contained information on 59 unique contraindicated drug combinations, only 68% of which were covered by any source. Lexi-Comp detected the most interactions (n = 29) and DRUG-REAX the least (n = 18). Only 3 drug combinations were detected and rated as contraindicated or potentially life-threatening in all 3 databases. The severity scores and level of documentation ratings varied widely. CONCLUSIONS: There are discrepancies among major drug interaction screening programs in the inclusion, severity, and level of documentation of contraindicated drug combinations mentioned in black box warnings. Further studies could explore the implications of these inconsistencies, particularly with regard to the integration of black box warning information in clinical practice. Clinicians should consult multiple drug resources to maximize the potential for detecting a potentially severe drug interaction.
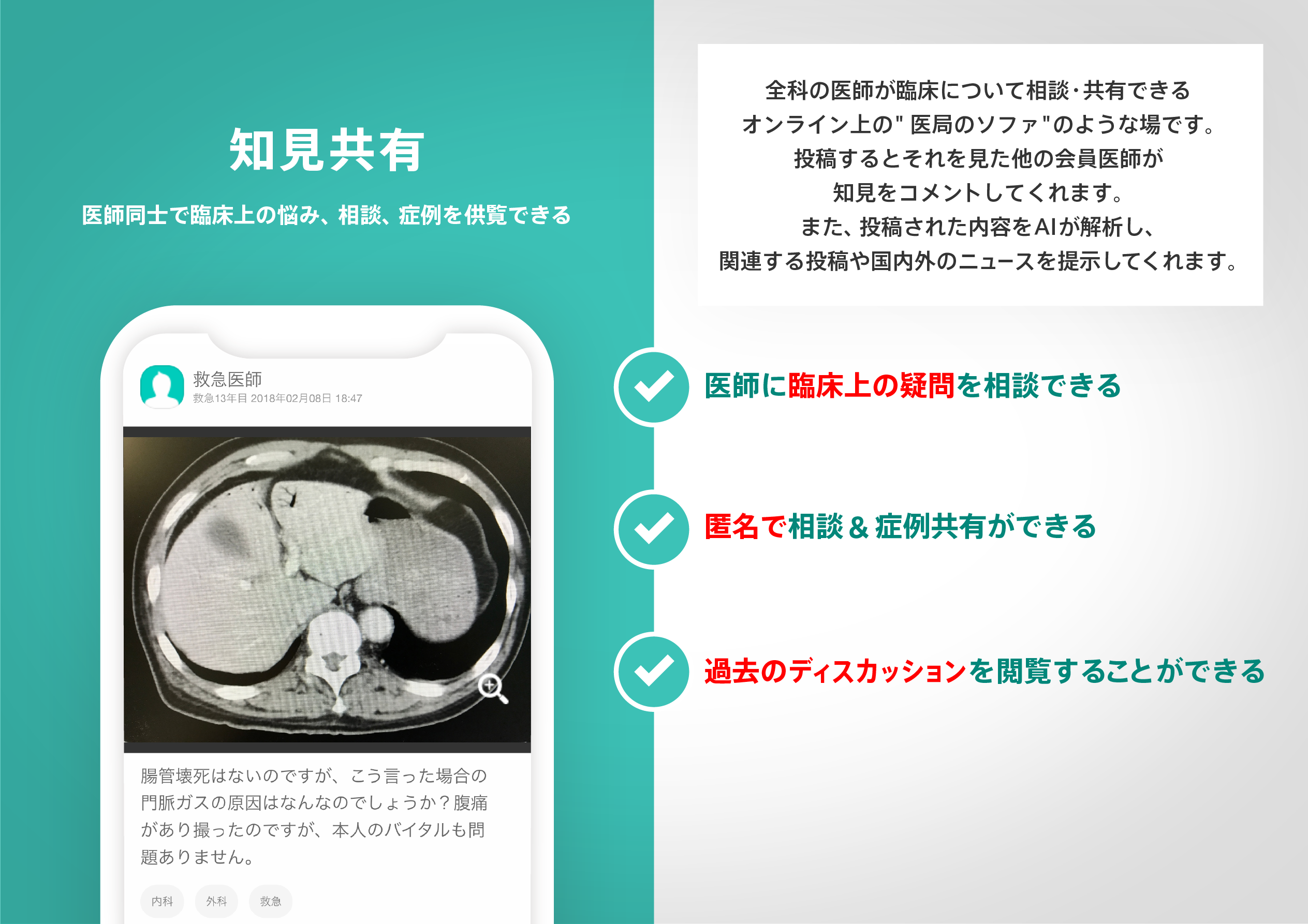
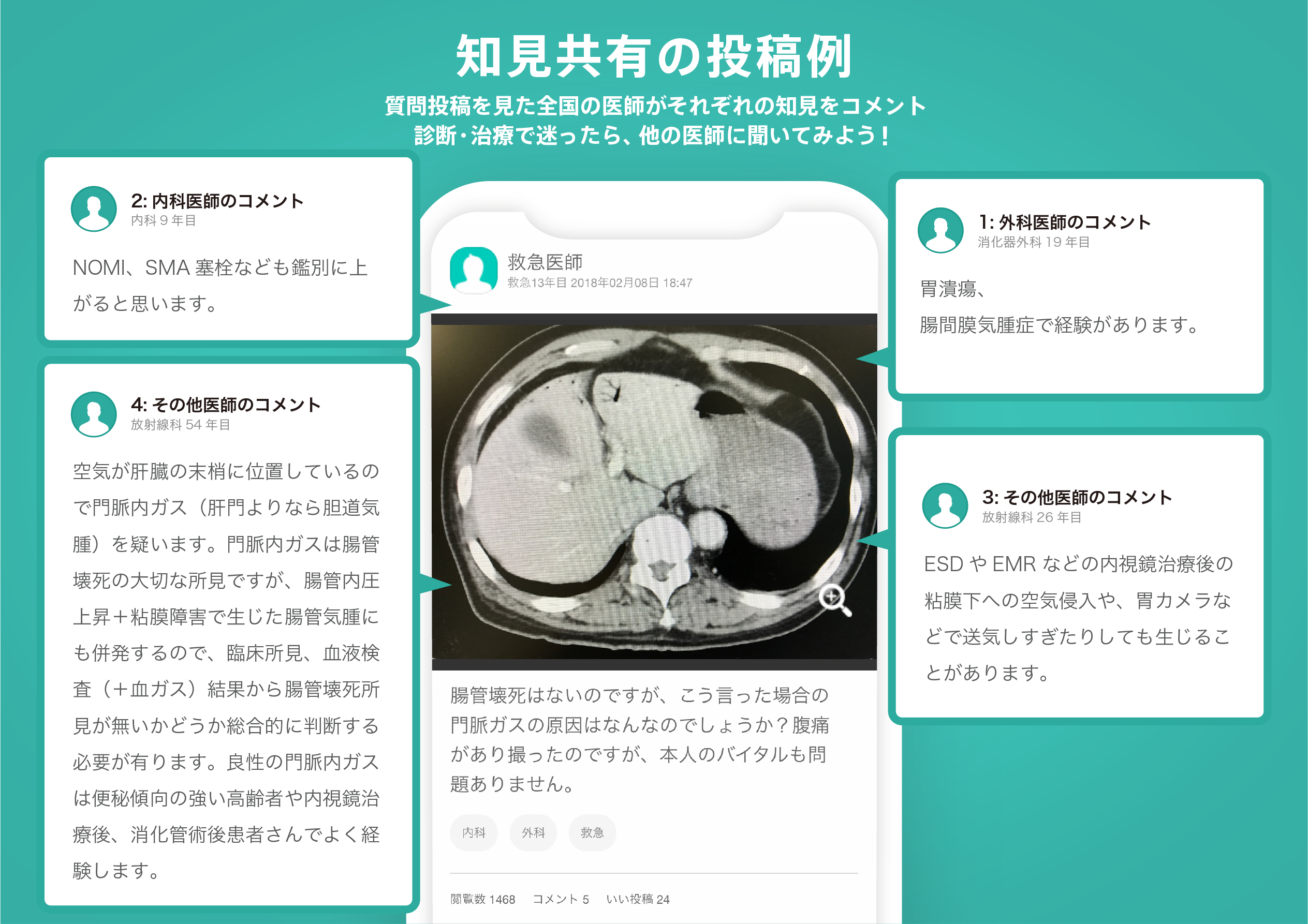
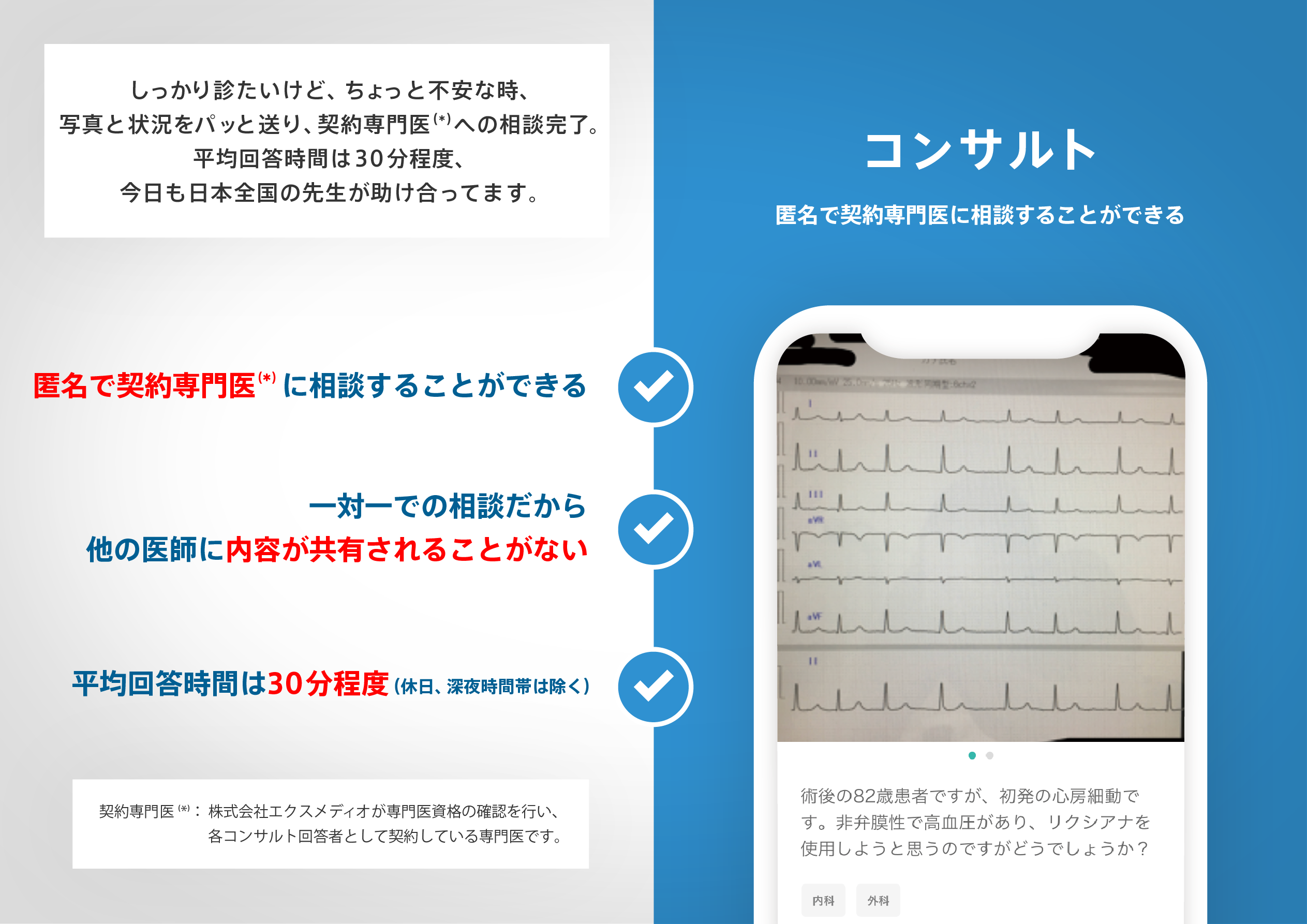
医師のための臨床サポートサービス
ヒポクラ x マイナビのご紹介
無料会員登録していただくと、さらに便利で効率的な検索が可能になります。