著名医師による解説が無料で読めます
すると翻訳の精度が向上します
This study was conducted to evaluate the utility of a selection of commercially and freely available non-testing tools and to analyse how REACH registrants can apply these as prioritisation tool for low-volume chemicals.分析は、広範なピアレビューされたリスク評価データを備えた有機工業化学物質と農薬のセットで実行されました。Analysed in silico model systems included Derek Nexus, Toxtree, QSAR Toolbox, LAZAR, TEST and VEGA, and results from these were compared with expert-judged risk classification according to the classifying, labelling and packaging (CLP) regulation.最も信頼できる結果は、発がん性について得られました。ただし、変異原性と生殖毒性については、信頼性の低い予測が導き出されました。A group of compounds frequently predicted as false negatives was identified.これらは、構造の複雑さが低い比較的小分子であり、たとえば、ヒドロキシル、アミノ、またはアニリン - 沈殿物を含むベンゼン誘導体でした。ラット肝臓S9代謝産物シミュレーターを適用して、リスク評価手順で代謝を考慮することの重要性を示しました。また、複数のモデルシステムからの予測を組み合わせることの結果についても説明し、シリコツールで適用する方法についてアドバイスします。These models are proposed to be used to prioritise low-volume chemicals for testing within the REACH legislation, and we conclude that further guidance is needed so that industry can select and apply models in a reliable, systematic and transparent way.
This study was conducted to evaluate the utility of a selection of commercially and freely available non-testing tools and to analyse how REACH registrants can apply these as prioritisation tool for low-volume chemicals.分析は、広範なピアレビューされたリスク評価データを備えた有機工業化学物質と農薬のセットで実行されました。Analysed in silico model systems included Derek Nexus, Toxtree, QSAR Toolbox, LAZAR, TEST and VEGA, and results from these were compared with expert-judged risk classification according to the classifying, labelling and packaging (CLP) regulation.最も信頼できる結果は、発がん性について得られました。ただし、変異原性と生殖毒性については、信頼性の低い予測が導き出されました。A group of compounds frequently predicted as false negatives was identified.これらは、構造の複雑さが低い比較的小分子であり、たとえば、ヒドロキシル、アミノ、またはアニリン - 沈殿物を含むベンゼン誘導体でした。ラット肝臓S9代謝産物シミュレーターを適用して、リスク評価手順で代謝を考慮することの重要性を示しました。また、複数のモデルシステムからの予測を組み合わせることの結果についても説明し、シリコツールで適用する方法についてアドバイスします。These models are proposed to be used to prioritise low-volume chemicals for testing within the REACH legislation, and we conclude that further guidance is needed so that industry can select and apply models in a reliable, systematic and transparent way.
This study was conducted to evaluate the utility of a selection of commercially and freely available non-testing tools and to analyse how REACH registrants can apply these as prioritisation tool for low-volume chemicals. The analysis was performed on a set of organic industrial chemicals and pesticides with extensive peer-reviewed risk assessment data. Analysed in silico model systems included Derek Nexus, Toxtree, QSAR Toolbox, LAZAR, TEST and VEGA, and results from these were compared with expert-judged risk classification according to the classifying, labelling and packaging (CLP) regulation. The most reliable results were obtained for carcinogenicity; however, less reliable predictions were derived for mutagenicity and reproductive toxicity. A group of compounds frequently predicted as false negatives was identified. These were relatively small molecules with low structural complexity, for example benzene derivatives with hydroxyl-, amino- or aniline-substituents. A rat liver S9 metabolite simulator was applied to illustrate the importance of considering metabolism in the risk assessment procedure. We also discuss outcome of combining predictions from multiple model systems and advise how to apply in silico tools. These models are proposed to be used to prioritise low-volume chemicals for testing within the REACH legislation, and we conclude that further guidance is needed so that industry can select and apply models in a reliable, systematic and transparent way.
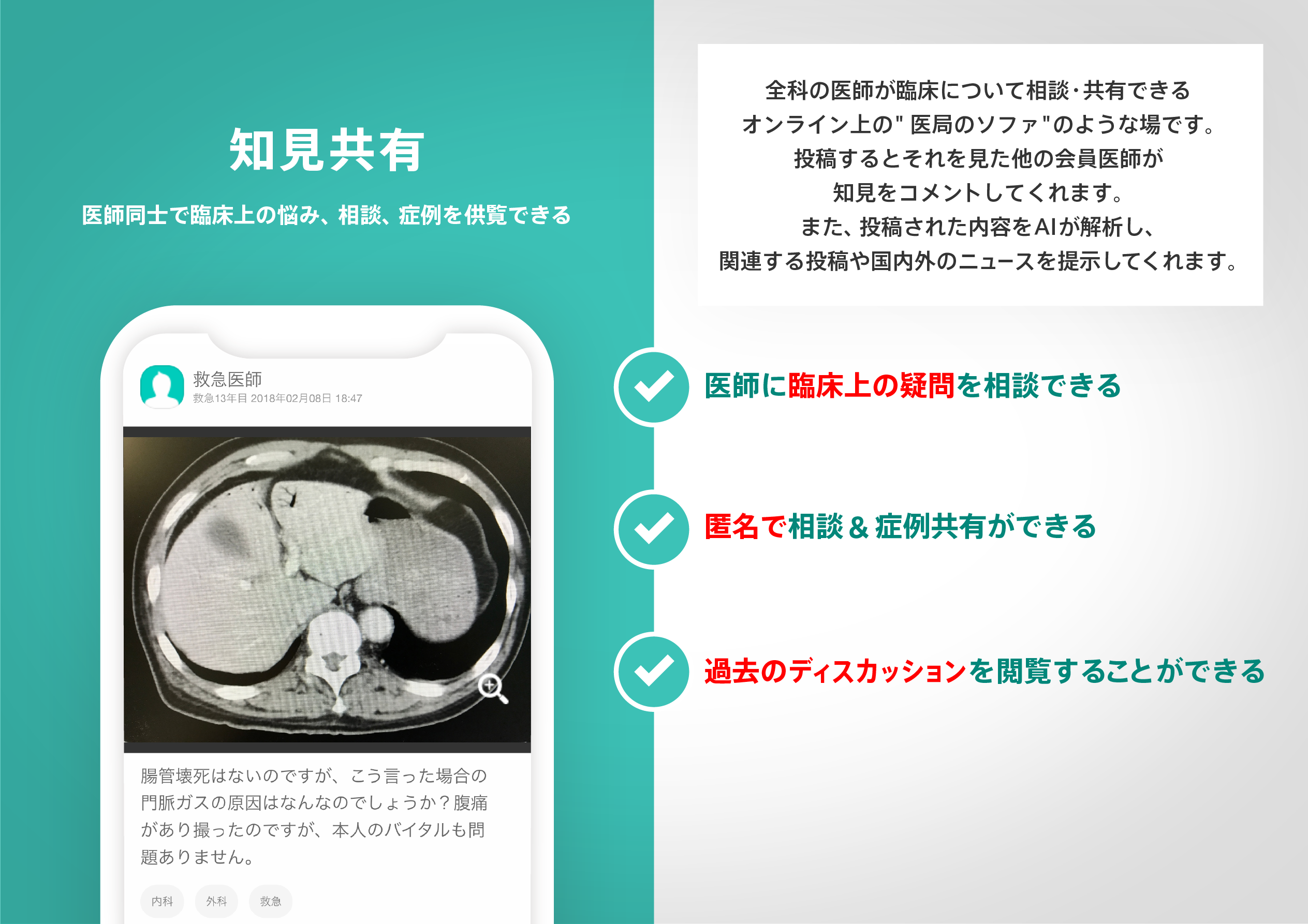
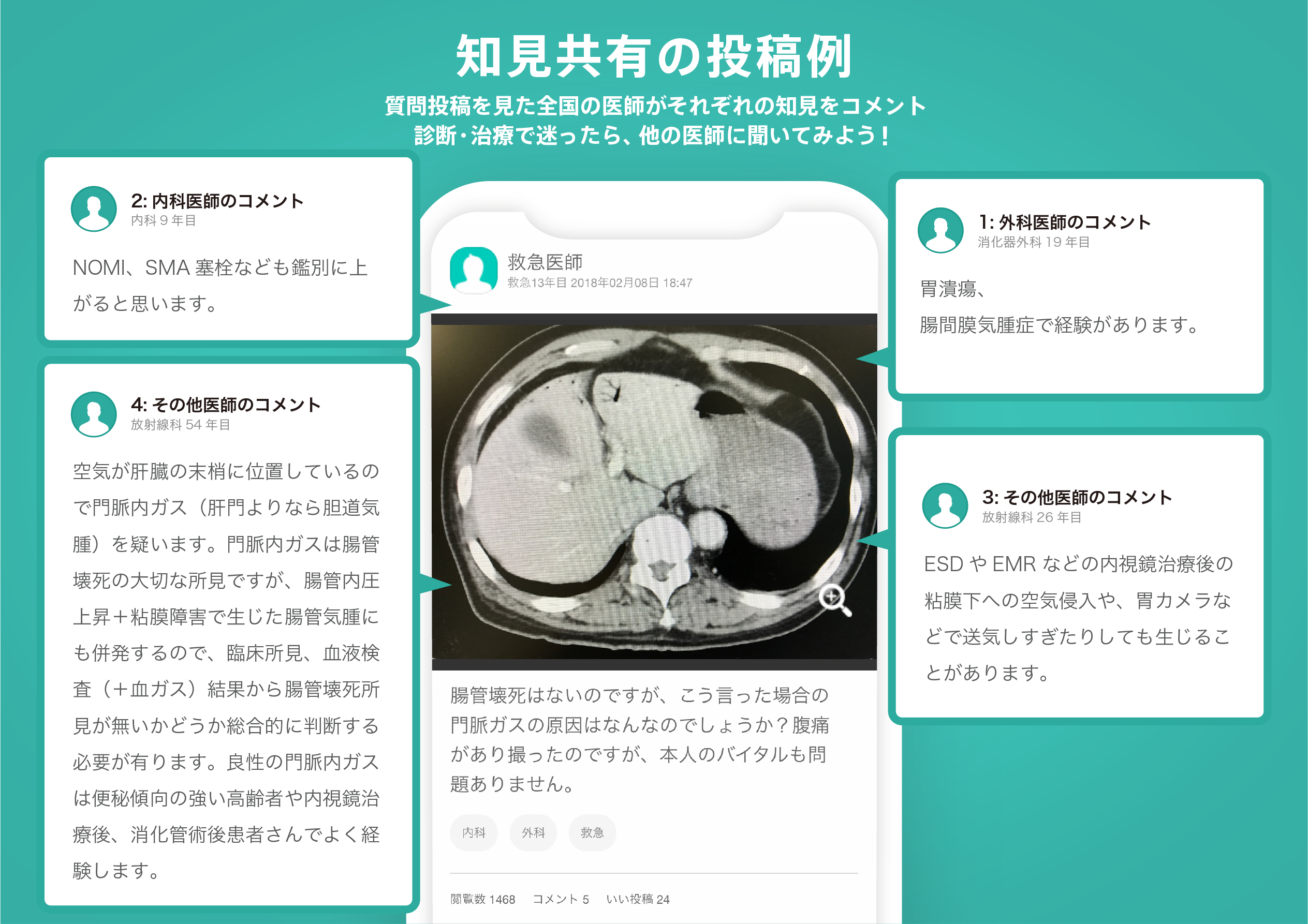
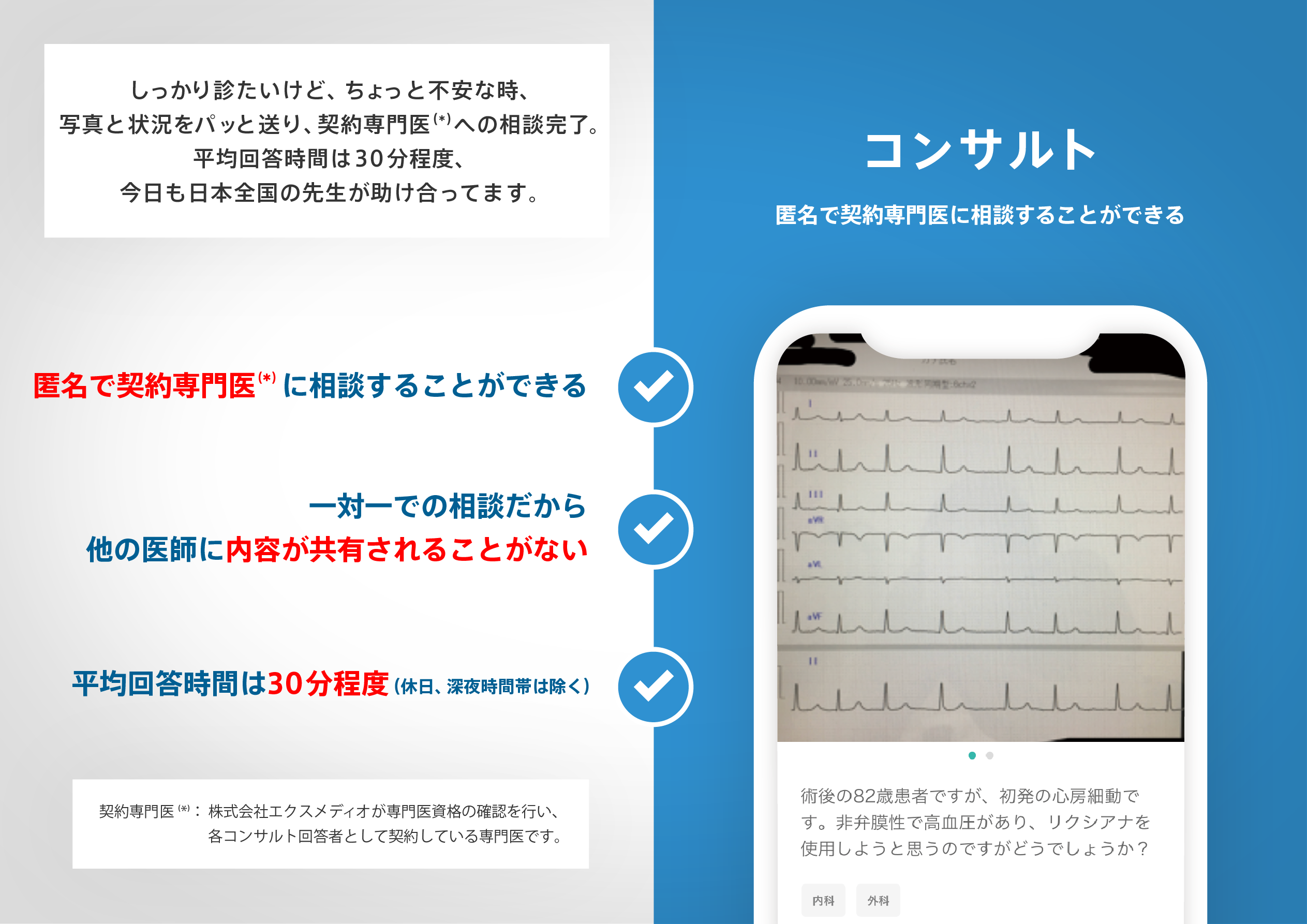
医師のための臨床サポートサービス
ヒポクラ x マイナビのご紹介
無料会員登録していただくと、さらに便利で効率的な検索が可能になります。